Updating the last IUPHAR News of 21/12/2021
Michael Spedding1, Anthony Davenport2 and Steve Alexander3,
1 IUPHAR and Spedding Research solutions SAS, 6 Rue Ampère, Le Vésinet 78110, France
2 University of Cambridge, Addenbrooke’s Centre for Clinical Investigation (ACCI), Addenbrooke’s Hospital, Cambridge, CB2 0QQ. U.K.
3 IUPHAR and University of Nottingham Medical School, Nottingham NG7 2UH, UK
Correspondence, michael@speddingresearchsolutions.fr;
The sudden appearance of the Omicron variant represents an enormous global health challenge, with such high infection rates that the WHO claimed that the majority of the World would be infected by end March. The subvariant BA.2 is about 1.5 times more transmissable that BA.1, and it is more adept at infecting people that are vaccinated or even boosted. However, it is clear that ensuing illness following Omicron infection appears to be less severe than the Delta variant, especially in vaccinated populations. However, mortality may still occur, albeit rarely in vaccinated individuals, and long-COVID may also result, so the massive infection rates may presage a chronic disease.
A remarkable web site, https://nextstrain.org/ncov, traces the evolution of the different strains of SARS-CoV-2. Millions of complete SARS-CoV-2 genomes have been reported and Figure 1. shows how quickly successive mutations outcompete each other. Omicron seems to behave in a similar way, rapidly becoming a dominant strain; in South Africa, the Omicron variant has outcompeted Delta, which has now virtually disappeared.
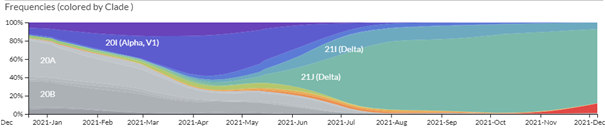
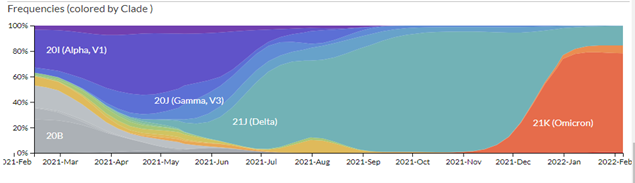
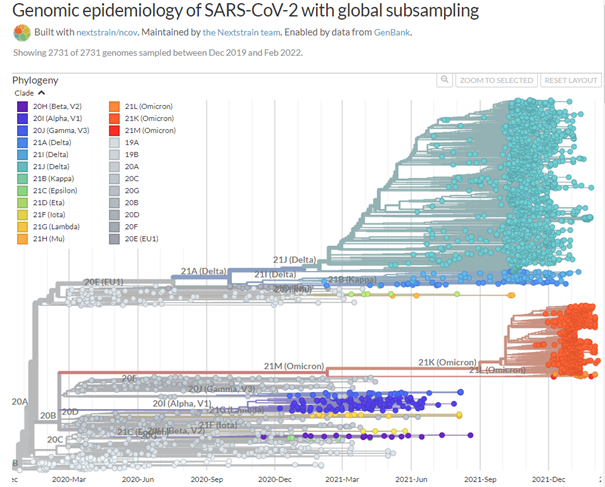
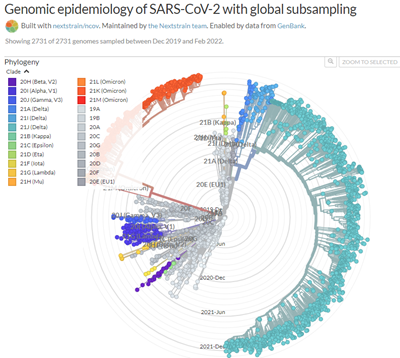
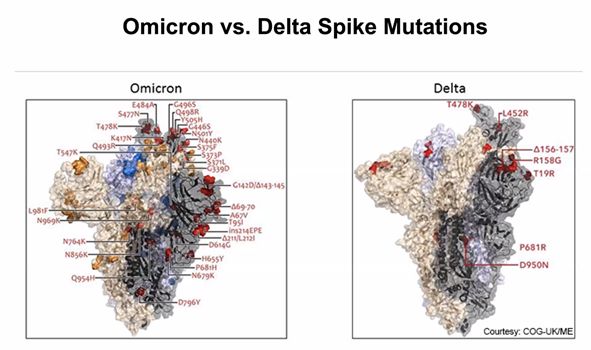
As we showed in the last update, the great number of mutations in the spike protein (Figure 3) facilitate interaction with the molecular target, the cell-surface enzyme and anchoring point ACE2, thereby increasing affinity with up to 84 mutations claimed (Ghosh et al., 2022). A controversy remains whether the mutations in the Spike protein were stable, or if they changed rapidly between substrains; resolution of this issue is important because flexibility in spike mutations could be serious for the design of future mRNA vaccines, and for sufficiently generalised immunity post infection. While nearly all discussion to date has centred on the mutations in spike protein (the receptor binding domain, RBD), other mutations are present and their impact on host response and on COVID-19 symptoms are still not yet clear. However, mutations in spike are used by the COVID-19 PCR test for tracking the spread of the Alpha (B.1.1.7) variant as the deletion in spike of amino acids 69 and 70 (Δ69–70) allows an S– indication where there are positive test results. The Delta variant is S+, so as Omicron is also S- and Alpha has been outcompeted, then where Omicron has been shown to be present by sequencing, the frequency of S- infection is taken as Omicron infection.
Spike changes account for increased infectivity by increasing the affinity of the S protein binding to the ACE2 protein, as well as increasing the efficiency of viral entry. Therefore the cocktail of anti-spike antibodies developed during the previous variants’ waves such as casirivimab, imdevimab and bamlanivimab may be less effective in protecting COVID-19 patients (Tatham L et al., 2022). However, it is also clear that children are readily infected by Omicron and may be responsible for at least part of the spread of the variant (Elliott et al., 2022).
At the last update, the key question was:
“whether Omicron will cause a pandemic of serious disease, or reflect a major reduction in the percentages of people with serious disease, and therefore a step towards adaptation to an endemic virus”. This question has been answered in that, while Omicron is capable of infecting vaccinated individuals, its toxic effects in the lung appear to be mainly restricted to the upper lung, rather than the lower lung toxicity of Delta, as shown by animal models (Halfmann et al., 2022; McMahan et al., 2022; Shuai et al., 2022). Clinically, there is less risk of severe disease or death in vaccinated individuals. Non-vaccinated, previously uninfected, subjects may still have a risk of severe disease, albeit less than with Delta, although evidently the infection rate is very high with Omicron (Fall et al., 2022; Robinson et al., 2022).
Thus, neutralizing antibody responses appear to be effective, if they are present in sufficient titres, but Omicron is the most resistant strain to date to current antibodies whether raised by vaccines or in response to infection by other strains (Chen et al., 2021).
However, SARS-CoV-2-specific CD8+ and CD4+ T cells, which respond to multiple targets, are also a robust defence after infection or vaccination (Grifoni et al., 2020). Keeton et al., 2022 showed that 70-80% of T cell responses were still conserved in vaccinated donors, and further bioinformatic analyses suggested that viral evolution was not escaping T cell responses. However, the ratio of different vaccines to evoke T cell responses, compared with antibodies, is still debated. David Montefiore showed convincing evidence of decline with time in antibody efficiency against Omicron infection (taking sera from vaccinated individuals and challenging the virus with progressive sera dilutions), but the booster injection maintained efficacy (Doria-Rose et al., 2021). As the CD8+-T cell responses are resistant to the changes in spike protein mutations then two vaccinations, especially with a booster, may still protect against severe disease.
Although the precise molecular mechanisms contributing to Long Covid remain to be elucidated, the pathophysiological consequences are becoming clear (Al-Aly et al., 2021), with damage to the cardiovascular system taking centre stage. For example, in a remarkable US study published in Nature, (Xie et al., 2022), a cohort of over 150, 000 individuals who had been infected with COVID-19 were compared with over 5 million contemporary and 5 million historical control individuals. The study showed that a year later, survivors of COVID-19 are at substantially increased risk of a range of cardiovascular diseases (including heart failure, pericarditis, myocarditis, dysrhythmias, ischemic and non-ischemic heart disease and cerebrovascular disorders) as well as blood clots (thrombosis). The risk increased according to the level of care in hospitalised patients during the acute phase of infection and but was evident even in non-hospitalised patients. In a preprint that included authors from the UK Office for National Statistics, a retrospective study reported that out of 48 000 patients hospitalised with COVID-19, nearly one third of individuals post COVID-19 hospital discharge were re-admitted and more than one in ten died (Ayoubkhani et al., 2021). Furthermore, the risks to mental health are also major (Xie et al., 2022).
Fortunately, we have one of the most accurate and rapid point of care diagnostic tools in the PCR and lateral flow tests, to accurately identify infected patients during the acute phase and likely to suffer from Long Covid. We are now moving into an era when bespoke pharmacological agents such as SARS-Covid-2 anti-virals are gaining approval, for treating the acute early phase but new variants may still escape these agents as well as the vaccines. Clearly, the endpoints being assessed for drug therapies, bearing in mind the risk of long COVID, will have to expand beyond the acute impact of infection. Pharmacologists should now be mobilising to conduct adequately powered clinical trials with existing cardiovascular (such as statins) and antithrombotic agents (such as oral anticoagulants) to improve the longer-term outcomes for patients who have been hospitalised with COVID-19. Although the rapid progress with vaccines has been remarkable, progress with finding drugs for treatment of COVID-19, has been much less successful, with 6billion$ being spent on clinical trials for many agents with limited chance of clinical success: more pharmacological input is needed in future pandemics in order to choose drugs to test. The re-purposing approach has realistically been limited to dexamethasone, thanks to the RECOVERY trial. It is beyond the scope of this short overview to review the drugs tested, but it is apparent that we need a full analysis, with wide ranging profiling of both host and viral pathogen, in order to allow the precision of understanding needed to allow optimal alignment of stratified populations with medication alternatives. We also need to ensure that pharmacologists are present in the relevant committees prioritising antiviral drugs.
In terms of the differential impact on subpopulations, an important study assessing 18457 pregnant women in Scotland showed that vaccine coverage was much lower in pregnant women than in the general female population of the same age group (32.3% having two doses of vaccine compared to 77.4%). However, there was a much higher perinatal mortality rate following COVID-19 diagnosis in poorly vaccinated women (22.6/1000) compared with control (5.6/1000) as well as an increase in baby deaths (Stock et al., 2022). Vaccination remains the essential resource in the pandemic.
The reason why children are more resistant to SARS-CoV-2 infection than adults has been elucidated in a study comparing single cell samples (nasal, tracheal, bronchial, blood) from children and adults before and during infection (Yoshida et al., 2022). Extensive differences were found between children and adults, as SARS-CoV-2 infection is antagonised by pre-stimulation of the interferon response, and children have a chronically stimulated interferon response. Furthermore, the interferon responses were different in children, with increases of naïve lymphocytes, and fewer natural killer cells, whereas in adults, cytotoxic T cells increased in an exacerbated interferon response.
The long term effects of the pandemic may affect healthcare durably. The effects of long COVID remain a source of disability, even if the molecular causes are obscure. However, envelope viruses, such as SARS-CoV-2, cause major changes to lipid metabolism, reducing oxidative phosphorylation which can be revealed by metabolomics (Gassen et al., 2021; see Junjhon et al., 2014; Chotiwan et al., 2018). In subjects with long-COVID following SARS-CoV-1, marked changes in lipid metabolism (such as acyl carnitine metabolism), were linked to poor functional recovery even 12 years after infection (Wu et al., 2017). Such large changes in lipid metabolism may be behind the reports of increased cardiac mortality following infection (Aghajani, 2022) and the major increases in stroke and heart failure incidence more than a month after infection (Xie et al., 2022). We have argued that the decline in VO2max seen with ageing may be directly associated with mitochondrial dysfunction caused by SARS-CoV-2 (Spedding et al, 2022). Furthermore, children, in particular, have suffered in lockdown from major reductions in play and education, the deleterious effects of which are only now becoming apparent.
Looking to the future, it must be remembered that a virus is not living, but rather a device that reproduces by rapid molecular roulette in host cells (eg Figure 2). A virus which can spread so effectively, especially at presymptomatic stages, is therefore not well biased to the long-term benefit of the host, so there may still be danger from future variants, especially if nations continue to allow very high infection rates, searching for ‘herd immunity’.
Al-Aly, Z., Xie, Y., & Bowe, B. (2021). High-dimensional characterization of post-acute sequelae of COVID-19. Nature, 594(7862), 259–264. https://doi.org/10.1038/s41586-021-03553-9
Ayoubkhani, D., Khunti, K., Nafilyan, V., Maddox, T., Humberstone, B., Diamond, I., & Banerjee, A. (2021). Epidemiology of post-COVID syndrome following hospitalisation with coronavirus: A retrospective cohort study. https://doi.org/10.1101/2021.01.15.21249885
Chen, J., Wang, R., Gilby, N. B., & Wei, G.-W. (2021). Omicron (B.1.1.529): Infectivity, vaccine breakthrough, and antibody resistance. ArXiv:2112.01318 [q-Bio]. http://arxiv.org/abs/2112.01318
Chotiwan, N., Andre, B. G., Sanchez-Vargas, I., Islam, M. N., Grabowski, J. M., Hopf-Jannasch, A., Gough, E., Nakayasu, E., Blair, C. D., Belisle, J. T., Hill, C. A., Kuhn, R. J., & Perera, R. (2018). Dynamic remodeling of lipids coincides with dengue virus replication in the midgut of Aedes aegypti mosquitoes. PLoS Pathogens, 14(2), e1006853. https://doi.org/10.1371/journal.ppat.1006853
Doria-Rose, N. A., Shen, X., Schmidt, S. D., O’Dell, S., McDanal, C., Feng, W., Tong, J., Eaton, A., Maglinao, M., Tang, H., Atmar, R. L., Lyke, K. E., Wang, L., Zhang, Y., Gaudinski, M. R., Black, W. P., Gordon, I., Guech, M., Ledgerwood, J. E., … Montefiori, D. C. (2021). Booster of mRNA-1273 Vaccine Reduces SARS-CoV-2 Omicron Escape from Neutralizing Antibodies (p. 2021.12.15.21267805). https://doi.org/10.1101/2021.12.15.21267805
Elliott, P., Bodinier, B., Eales, O., Wang, H., Haw, D., Elliott, J., Whitaker, M., Jonnerby, J., Tang, D., Walters, C. E., Atchison, C., Diggle, P. J., Page, A. J., Trotter, A. J., Ashby, D., Barclay, W., Taylor, G., Ward, H., Darzi, A., … Donnelly, C. A. (2022). Rapid increase in Omicron infections in England during December 2021: REACT-1 study. Science (New York, N.Y.), eabn8347. https://doi.org/10.1126/science.abn8347
Fall, A., Eldesouki, R. E., Sachithanandham, J., Paul Morris, C., Norton, J. M., Gaston, D. C., Forman, M., Abdullah, O., Gallagher, N., Li, M., Swanson, N. J., Pekosz, A., Klein, E. Y., & Mostafa, H. H. (2022). A Quick Displacement of the SARS-CoV-2 variant Delta with Omicron: Unprecedented Spike in COVID-19 Cases Associated with Fewer Admissions and Comparable Upper Respiratory Viral Loads. MedRxiv: The Preprint Server for Health Sciences, 2022.01.26.22269927. https://doi.org/10.1101/2022.01.26.22269927
Gassen, N. C., Papies, J., Bajaj, T., Emanuel, J., Dethloff, F., Chua, R. L., Trimpert, J., Heinemann, N., Niemeyer, C., Weege, F., Hönzke, K., Aschman, T., Heinz, D. E., Weckmann, K., Ebert, T., Zellner, A., Lennarz, M., Wyler, E., Schroeder, S., … Müller, M. A. (2021). SARS-CoV-2-mediated dysregulation of metabolism and autophagy uncovers host-targeting antivirals. Nature Communications, 12(1), 3818. https://doi.org/10.1038/s41467-021-24007-w
Ghosh, N., Nandi, S., & Saha, I. (2022). A review on evolution of emerging SARS-CoV-2 variants based on spike glycoprotein. International Immunopharmacology, 105, 108565. https://doi.org/10.1016/j.intimp.2022.108565
Grifoni, A., Weiskopf, D., Ramirez, S. I., Mateus, J., Dan, J. M., Moderbacher, C. R., Rawlings, S. A., Sutherland, A., Premkumar, L., Jadi, R. S., Marrama, D., de Silva, A. M., Frazier, A., Carlin, A. F., Greenbaum, J. A., Peters, B., Krammer, F., Smith, D. M., Crotty, S., & Sette, A. (2020). Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell, 181(7), 1489-1501.e15. https://doi.org/10.1016/j.cell.2020.05.015
Halfmann, P. J., Iida, S., Iwatsuki-Horimoto, K., Maemura, T., Kiso, M., Scheaffer, S. M., Darling, T. L., Joshi, A., Loeber, S., Singh, G., Foster, S. L., Ying, B., Case, J. B., Chong, Z., Whitener, B., Moliva, J., Floyd, K., Ujie, M., Nakajima, N., … Kawaoka, Y. (2022). SARS-CoV-2 Omicron virus causes attenuated disease in mice and hamsters. Nature. https://doi.org/10.1038/s41586-022-04441-6
Junjhon, J., Pennington, J. G., Edwards, T. J., Perera, R., Lanman, J., & Kuhn, R. J. (2014). Ultrastructural characterization and three-dimensional architecture of replication sites in dengue virus-infected mosquito cells. Journal of Virology, 88(9), 4687–4697. https://doi.org/10.1128/JVI.00118-14
Keeton, R., Tincho, M. B., Ngomti, A., Baguma, R., Benede, N., Suzuki, A., Khan, K., Cele, S., Bernstein, M., Karim, F., Madzorera, S. V., Moyo-Gwete, T., Mennen, M., Skelem, S., Adriaanse, M., Mutithu, D., Aremu, O., Stek, C., du Bruyn, E., … Riou, C. (2022). T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature. https://doi.org/10.1038/s41586-022-04460-3
McMahan, K., Giffin, V., Tostanoski, L. H., Chung, B., Siamatu, M., Suthar, M. S., Halfmann, P., Kawaoka, Y., Piedra-Mora, C., Martinot, A. J., Kar, S., Andersen, H., Lewis, M. G., & Barouch, D. H. (2022). Reduced Pathogenicity of the SARS-CoV-2 Omicron Variant in Hamsters (p. 2022.01.02.474743). bioRxiv. https://doi.org/10.1101/2022.01.02.474743
Robinson, M. L., Morris, C. P., Betz, J., Zhang, Y., Bollinger, R., Wang, N., Thiemann, D. R., Fall, A., Eldesouki, R. E., Norton, J. M., Gaston, D. C., Forman, M., Luo, C. H., Zeger, S. L., Gupta, A., Garibaldi, B. T., & Mostafa, H. H. (2022). Impact of SARS-CoV-2 variants on inpatient clinical outcome. MedRxiv: The Preprint Server for Health Sciences, 2022.02.02.22270337. https://doi.org/10.1101/2022.02.02.22270337
Shuai, H., Chan, J. F.-W., Hu, B., Chai, Y., Yuen, T. T.-T., Yin, F., Huang, X., Yoon, C., Hu, J.-C., Liu, H., Shi, J., Liu, Y., Zhu, T., Zhang, J., Hou, Y., Wang, Y., Lu, L., Cai, J.-P., Zhang, A. J., … Chu, H. (2022). Attenuated replication and pathogenicity of SARS-CoV-2 B.1.1.529 Omicron. Nature. https://doi.org/10.1038/s41586-022-04442-5
Stock, S. J., Carruthers, J., Calvert, C., Denny, C., Donaghy, J., Goulding, A., Hopcroft, L. E. M., Hopkins, L., McLaughlin, T., Pan, J., Shi, T., Taylor, B., Agrawal, U., Auyeung, B., Katikireddi, S. V., McCowan, C., Murray, J., Simpson, C. R., Robertson, C., … Wood, R. (2022). SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nature Medicine. https://doi.org/10.1038/s41591-021-01666-2
Tatham L, Sharp J, Kijak E, Herriott J, Neary M, Box H, Valentijn A, Cox H, Pertinez H, Curley P, Arshad U, Rajoli Rk, Rannard S, Stewart J, & Owen A. (2022). Lack of Ronapreve (REGN-CoV; casirivimab and imdevimab) virological efficacy against the SARS-CoV-2 Omicron variant (B.1.1.529) in K18-hACE2 mice. BioRxiv : The Preprint Server for Biology. https://doi.org/10.1101/2022.01.23.477397
Wu, Q., Zhou, L., Sun, X., Yan, Z., Hu, C., Wu, J., Xu, L., Li, X., Liu, H., Yin, P., Li, K., Zhao, J., Li, Y., Wang, X., Li, Y., Zhang, Q., Xu, G., & Chen, H. (2017). Altered Lipid Metabolism in Recovered SARS Patients Twelve Years after Infection. Scientific Reports, 7(1), 9110. https://doi.org/10.1038/s41598-017-09536-z
Xie, Y., Xu, E., & Al-Aly, Z. (2022). Risks of mental health outcomes in people with covid-19: Cohort study. BMJ (Clinical Research Ed.), 376, e068993. https://doi.org/10.1136/bmj-2021-068993
Yoshida, M., Worlock, K. B., Huang, N., Lindeboom, R. G. H., Butler, C. R., Kumasaka, N., Dominguez Conde, C., Mamanova, L., Bolt, L., Richardson, L., Polanski, K., Madissoon, E., Barnes, J. L., Allen-Hyttinen, J., Kilich, E., Jones, B. C., de Wilton, A., Wilbrey-Clark, A., Sungnak, W., … Meyer, K. B. (2022). Local and systemic responses to SARS-CoV-2 infection in children and adults. Nature, 602(7896), 321–327. https://doi.org/10.1038/s41586-021-04345-x